Dietary supplement

ENTitis® biotyk
Lozenges for children over 3 years old and adults for use during antibiotic therapy.
10 lozenges
Flavour:
What is ENTitis® Biotyk?
ENTitis® Biotyk is the ONLY product in Poland containing BIOTIC COMPLEX, a unique combination of two exceptional bacterial strains:
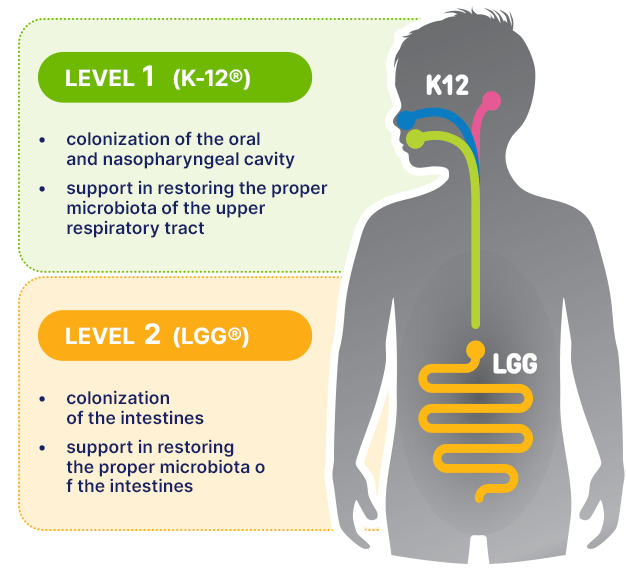
- Streptococcus salivarius K-12® in the Bactoblis® formula, which is active in the oral and nasopharyngeal cavity.
and - Lactobacillus rhamnosus (LGG®) by Chr.Hansen, which is active in the intestines.
These two distinct strains operate in different areas of our body, where they effectively colonize and strengthen two separate microbiomes.


Who is ENTitis® Biotyk for?
ENTitis® Biotyk is recommended for all children over 3 years old and adults, especially during antibiotic therapy, to support the microbiota of the oral cavity, nasopharynx, and intestines.
Most probiotic products available for use during antibiotic therapy contain bacterial strains that act exclusively in the intestines. However, when taking antibiotics, it is essential not only to support intestinal microbiota but also to maintain the balance of microbiota in the oral cavity and nasopharynx. Ensuring microbiotic balance in these two areas is crucial when antibiotic use is necessary.
How does ENTitis® Biotyk work?
The extensively studied K-12® strain in the Bactoblis® formula and LGG® are strains that actively work in two different areas of the body.
K-12® strain is active in the upper respiratory tract, colonizing the oral cavity and nasopharynx.
LGG® strain is active in the lower sections of the digestive tract, colonizing the intestines.
These strains work independently, supporting the body’s microbiome.

Why choose ENTitis® Biotyk?
The quality of bacterial strains used in products is crucial, as it affects the degree of microbiota colonization and, consequently, their effectiveness in the body. It is important to choose products whose original formulas have been tested in high-quality scientific studies. The highest quality and original complexes ensure proper effectiveness. Only ENTitis® Biotyk contains strains encapsulated in unique formulas, confirmed by over 300 scientific studies.
1 lozenge contains a total of 6 billion live bacterial cultures of Streptococcus salivarius K-12® and Lactobacillus rhamnosus (LGG®).
Ingredients
Filler: Isomalt; Lactobacillus rhamnosus (LGG®), Streptococcus salivarius K-12® (milk); flavouring: aroma; anti-caking agent: magnesium salts of fatty acids
| Content of active ingredients in the recommended daily serving – 1 lozenge: | |
| Streptococcus salivarius (K-12®) | 1 × 109 CFU* (1 billion) |
| Lactobacillus rhamnosus (LGG®) | 5 × 109 CFU* (5 billion) |
*CFU – colony-forming unit
Recommended daily serving
1 lozenge per day
10 days
1 lozenge per day during antibiotic therapy.
The lozenge should be taken before bedtime, after brushing your teeth. Slowly suck the lozenge for about 5 minutes until it is completely dissolved. Do not chew or swallow the lozenge. Do not drink anything immediately after consumption.
How to use ENTitis® Biotyk?


After completing antibiotic therapy, we recommend a 3-month use of the classic form of ENTitis®:
- For children over 3 years old: ENTitis® lozenges in strawberry or tutti frutti flavour.
- For adults: ENTitis® lozenges with a mint flavour.
Nearest pharmacy
Pharmacies near your location. See on the map where you can find our products.
Buy online
Choose your ENTitis®:

ENTitis®Baby
Sachets for infants over 6 months of age and children
DIETARY SUPPLEMENT
30 sachets with strawberry or banana flavour

ENTitis®
lozenges for children over 3 years of age and adults
DIETARY SUPPLEMENT
30 lozenges with strawberry flavour

ENTitis® biotyk
Lozenges for children over 3 years old and adults for use during antibiotic therapy.
DIETARY SUPPLEMENT
30 lozenges with tutti frutti flavor
ENTitis is the ONLY product in Poland containing the Streptococcus salivarius K-12® strain in the Bactoblis® formula, as well as Lactobacillus rhamnosus (LGG®). Bactoblis® is a slow-dissolving formula containing no less than 1 billion colony-forming units of the Streptococcus salivarius K-12® strain. Bactoblis® is a registered trademark of Pharmextracta S.P.A. and is exclusively licensed by Bluestone Pharma GmbH. LGG® and the LGG logo are registered trademarks of Chr. Hansen A/S.
**List of Scientific Studies for the K-12® Strain in the Bactoblis® Formula:
1. Carucci L., et al. Pediatr Allergy Immunol. 2022 Aug;33(8):e13836. 2. Damholt, A., et al (2022). Beneficial Microbes, 13(1), 13–23. 3. Groele L, et al. MJ Open Diabetes Res Care. 2021 Mar;9(1):e001523. 4. Adams CB, et al. BMC Pediatr. 2021 Jul 21;21(1):323. 5. Schnadower D, et al. J Nutr. 2021 Jan 4;151(1):65-72. 6. Mutlu M, et al. Am J Perinatol. 2020 Sep;37(11):1173-1176. 7. Hui, Y., et al (2021). Gut Microbes, 13(1). 8. Forster, C. S., et al. Forster, C. S., et al. 9. Schnadower, D., et al. Pediatr Gastroenterol Nutr, Aug 14 (2020). 10. Larnkjær, A., et al. Pediatric Research, (January), 1–6 (2020). 11. Castro-Mejía, J. L., et al. Gut Microbes, 00(00), 1–13 (2020). 12. Dawe, J. P., et al. Scientific Reports, 10(1), 1–11 (2020). 13. Groah et al. Therapeutic Advances in Urology, 11, 1–13, (2019). 14. Paparo et al, Scientific Reports, 9(1), 1–10, (2019). 15. Schmidt et al. Pediatr Allergy Immunol, 30(3), 1–6, (2019). 16. Bianchini et al. Human Vaccines & Immunotherapeutics, 0(00), 1–9, (2019). 17. Nabukeera-Barungi et al. Paediatrics and International Child Health, 39(2), 95–103, (2019). 18. Adler Sørensen et al. Clinical Microbiology and Infection, 25(4), 511.e1-511.e7, (2019). 19. Cabana et al. Journal of pediatric gastroenterology and nutrition, (2018). 20. Fjeldhøj et al. Scientific Reports, 8(1), 1–9, (2018). 21. Korpela et al. Microbiome, 6(1), 182, (2018). 22. Kallio et al. Clinical & Experimental Allergy. 2018. October. 23. Simpson et al. Journal of Dairy Science, 101(2), 889–899, (2018). 24. Kane et al. J Pediatr. 2018 Apr;195:73-79. 25. Berni Canani et al. Scientific Reports 8.1 (2018). 26. Lazarus et al. Vaccine, Jan 4;36(2):273-279, (2018). 27. Durack et al. Communications 9.1 (2018). 28. Schei, Kasper et al. Microbiome 5.1 (2017). 29. Narbona-López et al. British Journal of Nutrition, 117(7), 994 (2017). 30. Laursen et al. Pediatrics, 140(2), (2017). 31. Berni Canani et al. Allergy Clin Immunol. 2017 Jun;139(6):1906-1913. 32. Lundelin, et al. Pediatric Allergy and Immunology, 28(2), 170–175 (2017). 33. Grenov, et al. Journal of Pediatric Gastroenterology and Nutrition, 64(3), 396–403, (2017). 34. Cabana et al. Pediatrics. 2017 Sep;140(3). 35. Laursen et al. BMC Microbiol. 2017 Aug 17;17(1):175. 36. Rø et al. Clin Exp Allergy. 2017 Aug;47(8):1014-1021. 37. Groele et al. Open 7.10 (2017). 38. Peldan et al. Clinical & Experimental Allergy, 47(7), 975–979, (2017). 39. Scalabrin et al. Eur J Pediatr. 2017 Feb;176(2):217-224. 40. Schnadower et al. BMJ Open, 7(9), 1, (2017). 41. Fatheree, et al. World Journal of Gastrointestinal Pathophysiology, 7(1), 160. (2016). 42. Okesene-Gafa, et al. BMC Pregnancy Childbirth, 16(1):373 (2016). 43. Korpela et al. PLoS ONE, 11(4), 1–16, (2016). 44. Bruzzese, et al. Aliment.Pharmacol.Ther. 2016;44(6):568-575. 45. Okesene-Gafa, et al. BMC Pregnancy Childbirth, 16(1):373 (2016). 46. Fox, et al. BMJ Open 2015;5:1-6. 47. Ovcinnikova, et al. Clinicoecon Outcomes Res. 2015;7:145-152. 48. Dotterud, et al. J.Pediatr.Gastroenterol.Nutr. 2015;61(2):200-207. 49. Berni Canani, et al. Clin.Epigenetics 2015;7(1):38-015-0070-8. eCollection 2015. 50. Pärtty, et al. Pediatric Research, 78(4), 470–475, (2015). 51. Simpson, et al. BMC Dermatology, 15(1), 1–9, (2015). 52. Guest, et al. Clinicoecon Outcomes Res. 2015;7:583-591. 53. Berni Canani, et al. The ISME Journal, 10(3), 742–750, (2015). 54. Pärtty, et al. Pediatr Res, 77(6), 823–828, (2015). 55. Swanljung, et al. Acta Otolaryngol. 2015;135(8):824-830. 56. Aggarwal, et al. Indian J.Med.Res. 2014;139(3):379-385. 57. Luoto, et al. J.Allergy Clin.Immunol. 2014;133(2):405-413. 58. Sindhu, et al. Clin.Infect.Dis. 2014;58(8):1107-1115. 59. Tapiovaara, et al. Int.J.Pediatr.Otorhinolaryngol. 2014;78(10):1637-1641. 60. Havranek, et al. of Perinatology, 33(1), 40–44, (2013). 61. Kumpu, et al. J.Med.Virol. 2013;85(9):1632-1638. 62. Pärtty, et al. J.Pediatr. 2013;163(5):1272-1277.e2. 63. Berni Canani, et al. J.Pediatr. 2013;163(3):771-777. 64. Nylund et al. BMC Microbiology, 13, 12, (2013). 65. Glavina, et al. Antropol. 2012;36(1):129-132. 66. Hoppu, et al. Eur.J.Nutr. 2012;51(2):211-219. 67. Ou, et al. Clin.Exp.Allergy 2012;42(9):1386-. 68. Al-Hosni, et al. J.Perinatol. 2012:253-259.69. Pärtty, et al. PLoS One 2012;7(3):e32495-e32495. 70. Rautava, et al. Placebo-Controlled Trial Neonatology 2012;102(3):178-184. 71. Chrzanowska-Liszewska, et al. Early Hum Dev. 2012;88(1):57-60. 72. Nixon, et al. Pediatr.Emerg.Care 2012;28(10):1048-1051. 73. Kumpu, et al. European Journal of Clinical Nutrition, 66(9), 1020–1023, (2012). 74. Lehtoranta, et al. Int.J.Pediatr.Otorhinolaryngol. 2012;76:206-211. 75. Muraro, et al. BMJ Open 2012;2(2):e000637-. 76. Canani et al. Journal of Allergy and Clinical Immunology, 129(2), (2012). 77. Luoto, et al. Early Hum.Dev. 2012;88(6):339-344. 78. Grönlund, M. M., Grzeskowiak, Ł., Isolauri, E., & Salminen, S. (2011). Gut Microbes, 2(4). 79. Aaltonen, et al. Eur.J.Clin.Nutr. 2011;65:10-19. 80. Myhre, et al. Am.J.Clin.Nutr. 2011;93(8):151-157. 81. Rose, et al. Clin.Exp.Allergy 2011;41(12):1819-1821. 82. Ilmonen, et al. Clin.Nutr. 2011;30(2):156-164. 83. Nermes, et al. Clinical and Experimental Allergy, 41(3), 370–377, (2011). 84. McFarland et al. Education and Practice, 96(6), 238–238. (2010). 85. Cox, et al. PLoS One 2010:e8745. 86. Hojsak, et al. Pediatrics 2010;125:e1171. 87. Hojsak, et al. Clin.Nutr. 2010;29:312-316. 88. Baldassarre, et al. J.Pediatr. 2010;156:397-401. 89. Luoto, et al. Int.J.Obes. 2010;34:. 90. Salmi, et al. Pediatr. Allergy Immunol. 2010;21:e401. 91. Luoto et al. Acta Paediatrica, International Journal of Paediatrics, 99(8), 1135–1138, (2010). 92. Ritchie, et al. J.Pediatr.Gastroenterol.Nutr. 2010;50:619-624. 93. Dotterud, et al. Br.J.Dermatol. 2010;163:616-623. 94. Rose, et al. Clin.Exp.Allergy 2010;40:. 95. Luoto, et al. Br.J.Nutr. 2010;103(12):1792-1799. 96. Underwood et al. Journal of Pediatrics, The, 48(2), 216–225; (2009). 97. Rouge, et al. Am.J.Clin.Nutr. 2009;89:1828-1835. 98. Kuitunen, et al. J.Pediatr.Gastroenterol.Nutr. 2009;49:626-630. 99. Rautava, et al. Br.J.Nutr. 2009;101(11):1722-1726. 100. Kuitunen, et al. Journal of Allergy and Clinical Immunology, 123(2), 335–341 (2009). 101. Scalabrin, et al. Clin.Pediatr. 2009;48:734-744. 102. Aaltonen, et al. J.Pediatr. 2008;152:79-84. 103. Sentongo, et al. J.Pediatr.Gastroenterol.Nutr. 2008;46:41-47. 104. Marschan, et al. Clin.Exp.Allergy 2008;38:611-618. 105. Piirainen, et al. Ann.Nutr.Metab. 2008;52:204-208. 106. Kukkonen, et al. Pediatrics, 122(1), 8–12, (2008). 107. Huurre, et al. Clin.Exp.Allergy 2008;38:1342-1348. 108. Kloster Smerud, et al. Microb.Ecol.Health Dis. 2008;20:80-85. 109. Marschan, et al. Pediatr.Allergy Immunol. 2008;19:132-139. 110. Pediatr.Allergy Immunol. 2008;19:132-139. 111. Mentula, et al. Microb.Ecol.Health Dis. 2008;20(1):37-47. 112. Piirainen, et al. Ann.Allergy Asthma Immunol. 2008;100:338-342. 113. Kaplas, et al. Lipids 2007;42:865-870. 114. Grüber, et al. Allergy 2007;62(11):1270-1276. 115. Bruzzese, et al. Clin.Nutr. 2007;26:322-328. 116. Grönlund, et al. Clin.Exp.Allergy 2007;37(3):1764-1772. 117. Hatakka, et al. Clin.Nutr. 2007;26:314-321. 118. Kukkonen, et al. J.Allergy Clin.Immunol. 2007;119:192-198. 119. Honeycutt, et al. Pediatric Critical Care Medicine, 8(5), 452–458, (2007). 120. Kalliomäki et al. Journal of Allergy and Clinical Immunology, 119(4), 1018–1019; (2007). 121. Gueimonde, et al. J.Pediatr.Gastroenterol.Nutr. 2006;42:166-170. 122. Rinne, et al. J.Pediatr.Gastroenterol.Nutr. 2006;43:200-205. 123. Gueimonde, et al. J.Pediatr.Gastroenterol.Nutr. 2006;42:604-606. 124. Rautava, et al. Pediatr.Res. 2006;60:221-224. 125. Vendt, et al. J.Hum.Nutr.Diet. 2006;19:51-58. 126. Galpin, et al. Am.J.Clin.Nutr. 2005;82:1040-1045. 127. Viljanen, et al. Allergy 2005;60:494-500. 128. Bousvaros, et al. Inflamm.Bowel Dis. 2005;11:833-839. 129. Petschow, et al. J.Clin.Gastroenterol. 2005;39:786-790. 130. Laitinen, et al. Br.J.Nutr. 2005;94:565-574. 131. Bausserman, Michail. J.Pediatr. 2005;147:197-201. 132. Viljanen, et al. Pediatr.Allergy Immunol. 2005;16:65-71. 133. Rinne, et al. J.Pediatr. 2005;147:186-191. 134. Viljanen, et al. Allergy Clin.Immunol. 2005;115:1254-1259. 135. Schultz, et al. J.Pediatr.Gastroenterol.Nutr. 2004;38:293-297. 136. Salazar-Lindo, et al. BMC Pediatrics, 4(1), 1–9, 2004. 137. Pohjavuori, et al. J.Allergy Clin.Immunol. 2004;114:131-136. 138. Agarwal, et al. J.Pediatr.Gastroenterol.Nutr. 2003;36:397-402. 139. Kirjavainen, et al. Journal of Pediatric Gastroenterology and Nutrition, 36(2), 223–227; 2003. 140. Costa-Ribeiro, et al. J.Pediatr.Gastroenterol.Nutr. 2003;36:112-115. 141. Kalliomaki, et al. Lancet 2003;361:1869-1871. 142. Rautava et al. J Allergy Clin Immunol 2002;109:119-21. 143. Helin, et al. Allergy 2002;57:243-246. 144. Rautava, et al. J.Allergy Clin.Immunol. 2002;109:119-121. 145. Kankaanpää, P. E., Yang, B., Kallio, H. P., Isolauri, E., & Salminen, S. J. (2002). The Journal of nutritional biochemistry, 13(6), 364–369. 146. Hatakka, et al. BMJ 2001;322(7298):1327. 147. Näse, et al. Caries Res. 2001;35(6):412-420. 148. Kalliomaki, et al. Lancet 2001;357(9262):1076-1079. 149. Gupta, et al. J.Pediatr.Gastroenterol.Nutr. 2000;31:453-457. 150. Isolauri, et al. Clin.Exp.Allergy 2000;30(11):1604-1610. 151. Pessi, et al. Clin.Exp.Allergy 2000;30(12):1804-1808. 152. Vanderhoof, et al. West.J.Med. 2000;173:397. 153. Oberhelman, et al. J.Pediatr. 1999;134:15-20. 154. Arvola, et al. Pediatrics 1999;104:e64. 155. Vanderhoof, et al. J.Pediatr. 1999;135:564-568. 156. Rautanen, et al. Arch.Dis.Child. 1998;79:157-160. 157. Majamaa, Isolauri. J.Allergy Clin.Immunol. 1997;99:179-185. 158. Shornikova, et al. Acta Paediatr. 1997;86:460-465. 159. Gronlund, et al. Acta Paediatr, 86(4), 440–441, (1997). 160. Malin, et al. Inflammopharmacology 1997:219-236. 161. Malin, et al. Br.J.Rheumatol. 1996;35:689-694. 162. Malin, et al. Ann.Nutr.Metab. 1996;40:137-145. 163. Isolauri, et al. Vaccine 1995;13:310-312. 164. Majamaa, et al. J.Pediatr.Gastroenterol.Nutr. 1995;20:333-338. 165. Kaila, et al. Arch.Dis.Child. 1995;72:51-53. 166. Isolauri, et al. Dig.Dis.Sci. 1994;39(12):2595-2600. 167. Stansbridge, et al. Arch.Dis.Child. 1993;69:488-492. 168. Millar, et al. Arch.Dis.Child. 1993;69:483-487. 169. Kaila, et al. Pediatr.Res. 1992;32:141-144. 170. Isolauri, et al. Pediatrics 1991;88:90-97. 171. Oksanen, et al. Ann.Med. 1990;22:53-56. 172. Aljumaah MR., et al. Clin Nutr. 2022 Nov;41(11):2565-2576. 173. Mahboobi S, et al. Front Nutr. 2022 Sep 28;9:1018357. 174. Rauseo AM, et al. Infect Control Hosp Epidemiol. 2022 Feb;43(2):167-173. 175. Tractenberg RE, et al. PM R. 2021 Jul;13(7):695-706. 176. Johnstone, J., et al. JAMA – Journal of the American Medical Association, 326(11), 1024–1033. 177. Castro-Herrera VM, et al. Front Immunol. 2021 Mar 4;12:643321. 178. Forster, C. S., et al. J Spinal Cord Med. 2021 Jan;44(1):62-69. 179. Bornholdt, J., et al. Gut Microbes. 2020 Nov 9;12(1):1-14. 180. Butler, C. C., et al. JAMA – Journal of the American Medical Association, 324(1), 47–56 (2020). 181. Sanborn, V., et al. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 14(5), 907–909 (2020). 182. Dawe, J. P., et al. Scientific Reports, 10(1), 1–11 (2020). 183. Sanborn, V., Azcarate-Peril, M. A., Updegraff, J., Manderino, L., & Gunstad, J. (2020). Neuropsychiatric disease and treatment, 16, 2765–2777. 184. Toh, S., et al. Spinal Cord, 1–13. (2020). 185. Bianchini et al. Human Vaccines & Immunotherapeutics, 0(00), 1–9, (2019). 186. Okesene-Gafa et al. American Journal of Obstetrics and Gynecology, 221(2), 152.e1-152.e13 (2019). 187. Forster et al. Journal of Spinal Cord Medicine, 0(0), 1–8, (2019). 188. Seppo et al. JAMA Pediatrics, 1–3, (2019). 189. Wang et al. Journal of the American Geriatrics Society, 66(7), 1346–1352, (2018). 190. Simpson et al. Journal of Dairy Science, 101(2), 889–899, (2018). 191. Dickerson et al. Bipolar Disorders, 1–8, (2018). 192. Harata et al. European Journal of Nutrition, 56(7), 2245–2253, (2017). 193. Poutsiaka et al. Journal of Applied Microbiology, 122(5), 1321. (2017). 194. Burton et al. British Journal of Nutrition, 117(9), 1312–1322, (2017). 195. Gorshein et al. International Journal of Laboratory Hematology, 31(5), (2017). 196. Costabile et al. Frontiers in Immunology, 8, 1–13 (2017). 197. Kalima, et al. Beneficial Microbes, 7(4), 463–471, (2016). 198. Gueimonde, et al. Food Funct., 7(3), 1601–1609, (2016). 199. Tapiovaara, et al. International Forum of Allergy and Rhinology, 6(8), 848–853, (2016). 200. Cook, et al. Trials, 17, 377 (2016). 201. Solano-Aguilar, et al. PLoS One 2016;11(2):e0147426. 202. Kolmeder et al. PLoS ONE, 11(4), 1–23, (2016). 203. Doron, et al. Antimicrob.Agents Chemother. 2015;59(8):4593-4599. 204. Kwon, et al. Infect.Control Hosp.Epidemiol. 2015;36(12):1451-1454. 205. Tomasik, et al. Biomarker Insights, 10, 47–54, (2015). 206. Hauser, et al. Medicine (Baltimore) 2015;94(17):e685-. 207. Kumpu, et al. Benef Microbes 2015;6(5):631-639. 208. Eloe-Fadrosh, et al. MBio, 6(2), 1–12, (2015). 209. Hoppu, et al. Nutrition 2014;30(2):159-164. 210. Lehtoranta, et al. J.Clin.Virol. 2014;60(3):276-281. 211. Bajaj, et al. Aliment.Pharmacol.Ther. 2014;39(10):1113-1125. 212. Hibberd, et al. PLoS One 2014;9(12):e113456. 213. Dickerson, et al. Prim.Care.Companion CNS Disord. 2014;16(1):. 214. Smith, et al. Br.J.Nutr. 2013;109(11):1999-2007. 215. Nitert, et al. BMC Pregnancy Childbirth 2013;13:50-2393-13-50. 216. Lahti et al. PeerJ, 2013(1), 1–25. 217. Granata, et al. Int.J.Food Sci.Nutr. 2013;64(2):162-168. 218. Kumpu, et al. Br.J.Nutr. 2013;109(12):2240-6. 219. Välimäki, et al. Int.J.Sports Med. 2012;33(4):291-296. 220. Luoto, et al. Early Hum.Dev. 2012;88(6):339-344. 221. Ou, et al. Clin.Exp.Allergy 2012;42(9):1386-1396. 222. Hoppu, et al. Eur.J.Nutr. 2012;51(2):211-219. 223. West, et al. Gut Microbes 2012;3(3):. 224. van Baarlen, et al. Proceedings of the National Academy of Sciences, 108(Supplement_1), 4562–4569, (2010). 225. Ilmonen, et al. Clin.Nutr. 2011;30(2):156-164. 226. Kubota, et al. Curr.Microbiol. 2010;62. 227. Ferrie et al. Journal of Parenteral and Enteral Nutrition, 35(1), 43–49, (2011). 228. Kekkonen, et al. Journal of Nutrition, 141(5), 870–876, (2011). 229. Davidson, et al. Eur.J.Clin.Nutr. 2011;65(4):501-507. 230. Myhre, et al. Am.J.Clin.Nutr. 2011;93(8):151-157. 231. Frech, et al. Clin.Exp.Rheumatol. 2011;29(2 Suppl 65):S22. 232. Brantsćter, et al. Am.J.Epidemiol. 2011;174(7):807-815. 233. Holma, et al. J.Nutr. 2010;140:534-541. 234. Saxelin, et al. Int.J.Food Microbiol. 2010;144:. 235. Barraud, et al. Intensive Care Med. 2010;36(9):1540-1547. 236. Lyra et al. BMC Gastroenterology, 10, (2010). 237. Morrow, et al. Am.J.Respir.Crit.Care Med. 2010;182:. 238. Dommels, et al. Appl.Environ.Microbiol. 2009;75(19):6198-6204. 239. Kubota, et al. Microbiol.Immunol. 2009;53:198-205. 240. Laitinen, et al. Br.J.Nutr. 2009;101(11):1679-1687. 241. Kawase, et al. Int.J.Food Microbiol. 2009;128:429-434. 242. Kawase, et al. Int.J.Probiotics Prebiotics 2009:241-248. 243. Kekkonen, et al. World J.Gastroenterol. 2008;14(13):2029-2036. 244. Wenus, et al. Eur.J.Clin.Nutr. 2008;62:299-301. 245. Kekkonen, et al. World J.Gastroenterol. 2008;14(20):3188-3194. 246. Piirainen, et al. Ann.Allergy Asthma Immunol. 2008;100:338-342. 247. Osterlund, et al. Br.J.Cancer 2007;97:1028-1034. 248. Kekkonen, et al. Int.J.Sport Nutr.Exerc.Metab. 2007;17:352-363. 249. Hatakka, et al. J.Dent.Res. 2007;86:125-130. 250. Myllyluoma, et al. Dig.Liver Dis. 2007;39:516-523. 251. Cohen, et al. In Vivo 2007;21:507-512. 252. Roller, et al. Br.J.Nutr. 2007;97:676-684. 253. Myllyluoma, et al. Int.J.Antimicrob.Agents 2007;29:66-72. 254. Moreira, et al. Respir.Med. 2007;101:1123-1131. 255. Larkin, et al. Nutrition 2007;23(10):709-718. 256. Rafter, et al. Am.J.Clin.Nutr. 2007;85:488-496. 257. Kekkonen RA, et al. Milchwissenschaft 2007;62:326-330. 258. Manley, et al. Med.J.Aust. 2007;186:454-457. 259. Yli-Knuuttila, et al. Oral Microbiol.Immunol. 2006;21:129-131. 260. Hongisto, et al. Eur.J.Clin.Nutr. 2006;60:319-324. 261. Kajander, Korpela. Asia Pac.J.Clin.Nutr. 2006;15:576-580. 262. Kajander, et al. Aliment.Pharmacol.Ther. 2005;22:387-394. 263. Myllyluoma, et al. Aliment.Pharmacol.Ther. 2005;21(10):1263-1272. 264. Devillard, et al. Infect.Dis.Obstet.Gynecol. 2005;13:25-31. 265. de Vrese, et al. Eur.J.Nutr. 2005;44:406-413. 266. Schultz, et al. BMC Gastroenterology, 4, 3–6, (2004). 267. Salminen, et al. HIV.Clin.Trials 2004:183-191. 268. Gosselink, et al. Dis.Colon Rectum 2004;47:876-884. 269. Hatakka, et al. Scand.J.Rheumatol. 2003;32:211-215. 270. Gluck, Gebbers. Am.J.Clin.Nutr. 2003;77:517-520. 271. Kuisma, et al. Aliment.Pharmacol.Ther. 2003;17:509-515. 272. Colodner, et al. Israel Medical Association Journal, 5(11), 767–769; 2003. 273. Schultz, et al. J.Dairy Res. 2003;70(2):165-173. 274. Helin, et al. Allergy 2002;57:243-246. 275. Ahola, et al. Arch.Oral Biol. 2002;47(11):799-804. 276. Mirasoli, et al. Minerva Gastroenterol.Dietol. 2002;48:45-49. 277. Kontiokari, et al. BMJ 2001;322(7302):1571. 278. Gotteland, et al. Aliment.Pharmacol.Ther. 2001;15:11-17. 279. Apostolou, et al. FEMS Immunol.Med.Microbiol. 2001;30:217-221. 280. Thomas, et al. Mayo Clin.Proc. 2001;76:883-889. 281. Fang, et al. FEMS Immunol.Med.Microbiol. 2000;29:47-52. 282. Alander, et al. Appl.Environ.Microbiol. 1999;65:351-354. 283. Pelto, et al. Clin.Exp.Allergy 1998;28(12):1474-1479. 284. Hilton, et al. J.Travel Med. 1997, 4(1) :41-43. 285. Alander, et al. Lett.Appl.Microbiol. 1997;24:361-364. 286. Bennet, et al. Nutrition today supplemenmt. 1996;31(6):35S-38S. 287. Saxelin, et al. Int.J.Food Microbiol. 1995;25(2):199-203. 288. Ling, et al. J.Nutr. 1994;124:18-23. 289. Meurman, et al. Microb.Ecol.Health Dis. 1994:295-298. 290. Saxelin, et al. Microb.Ecol.Health Dis. 1993:119-122. 291. Goldin, et al. Dig.Dis.Sci. 1992;37:121-128. 292. Ling, et al. Ann.Nutr.Metab. 1992;36:162-166. 293. Saxelin, et al. Microb.Ecol.Health Dis. 1991:209-214. 294. Oksanen, et al. Ann.Med. 1990;22:53-56. 295. Siitonen, et al. Ann.Med. 1990;22:57-59. 296. Gorbach, et al. 1987(8574):1519. 297. Di Pierro F. et al. Int J General Medicine, 2012:5, 991–997. 298. Di Pierro F. et al. Clinical Evaluation: Streptococcus salivarius K12 for Recurrent Pharyngitis Prevention in Adults, 2013, 1-5. 299. Di Pierro F. et al. Drug, Healthcare and Patient Safety, 2014:6, 15–20. 300. Di Pierro F. et al. Int J General Medicine, 2015:8, 303-308. 301. Gregori G. et al. Therapeutics and Clinical Risk Management, 2016:12, 87-92. 302. Di Pierro F. et al. Drug, Healthcare and Patient Safety, 2016:8, 77-81. 303. Di Pierro F. et al. Eur. Rev. Med. Pharm. Sci.,2016:20, 4601-4606. 304. Gun T. Paripex Indian J. Res., 2017, 633-634. 305. Kryuchko T.O., Tkachenko O.Ya., Ukrainian Med. Dent. Acad., Lantibiotics for Preventing Recurrent Upper Respiratory Tract Infections in Children, 2017, 27-32.306. Di Pierro F. et al. Edizioni Minerva Med., 2018, 70(3):240-245. 307. Gavrilenko Yu.V., Prevention of Recurrent Pharyngeal Infections in Children with Antibiotics, 2018, 82-94. 308. Marushko T.V. et al. Streptococcus salivarius K12 in Preventing and Treating Chronic Tonsillitis in Children, 2018, 82-88. 309. Kruchko T.O., Tkachenko O. Ya. Clinical Use of Streptococcus salivarius K12 for Preventing Pharyngotonsillitis and Respiratory Infections in Children, 2018, 122-127. 310. Marini G. et al. Int J General Medicine, 2019:12, 213-217. 311. Havrylenko Yu.V. Clinical Use of Respiratory Probiotic Bactobolis in Children with Secretory Otitis Media, 2019, 90-93. 312. Ilchenko S.I. et al. Pediatrics. Eastern Europe, 2019, 559-565. 313. Bezshapochny S.B. et al. Optional Location Therapy for Chronic Tonsillopharyngitis Remission, Otorhinolaryngology 3(3), 2020, 38-42. 314. Sarlin S. et al.The Pediatr. Infect. Dis. J. 2020;XX:00-00, 1-9. 315. Karakov K. G. et al. Actual problems in dentistry, 2020, 18-21. 316. Kramarev S. O. et al. Actual Infectology, Application of S. salivarius K12 Probiotic in Acute Tonsillopharyngitis Treatment in Children. 2020, 6-11. 317. Ilchenko S.I. et al. Actual Infectology, Efficiency of Respiratory Probiotic S. salivarius K12 in Children with Recurrent Tonsillitis, 2020, 35-39. 318. Kryuchko T.O., Tkachenko O.Ya. Nutrafoods, 2021, 1:246-253. 319. Kryuchko T.O., Tkachenko O.Ya. Nutrafoods, 2021, 2:254-261. 320. Savlevich E.L. et al. Vestnik otorinolaringologii, Vol. 86, No. 6, 2021, 42-47. 321. Puhlik S.M. et al. Experience with Oral Probiotic S. salivarius K12 for Preventing Recurrent Pharyngotonsillar Episodes, 2021, 120-124. 322. Wang Q. et al. Oropharyngeal Probiotic ENT-K12 Prevents Respiratory Tract Infections Among Frontline Medical Staff Fighting Against COVID-19: A Pilot Study, 2021. 323. Di Pierro F. et al. Minerva Medica 2021, 112(4):514-6. 324. Kryuchko T., Tkachenko O. et al. Pediatrics. Eastern Europe, 2021; 9(3), 482-491. 325. Guo H. et al. Oropharyngeal Probiotic ENT-K12 as an Effective Dietary Intervention for Children With Recurrent Respiratory Tract Infections During Cold Season, 2022. 326. Babina K. et al. A Pilot Random. Clinical Trial. Nutrients, 2022, 7;14(5):1124. 327. A.Y. Ovchinnikov et al. Possibilities of probiotic therapy in chronic inflammatory diseases of the oropharynx. Effective pharm. 2022; 18 (4): 24–28. 328. Zangrilli A. et al. Probiotics and Antimicrobial Proteins, 2022, 14:573–578. 329. Di Pierro F. et al. Microorganisms, Clinical Effects of Streptococcus salivarius K12 in Hospitalized COVID-19 Patients: Results of a Preliminary Study 2022; 10(10):1926. 330. La Torre F. et al. Preliminary data revealing efficacy of SSK12 in Periodic Fever, Aphthous stomatitis, Pharyngitis, and cervical Adenitis (PFAPA) syndrome: A multicenter study from the AIDA Network PFAPA syndrome registry, 2023. 331. Bertuccioli A. et al. Use of.
*** The properties of the product result from the action of the ingredients. ENTitis® contains the Streptococcus salivarius K-12® strain in the Bactoblis® formula and vitamin D, which helps in the proper functioning of the immune system. Bactoblis® is a registered trademark of Pharmextracta S.P.A. and licensed exclusively by Bluestone Pharma GmbH. Bactoblis® is a product in the form of a slowly dissolving formula containing no less than 1 billion colony forming units of the Streptococcus salivarius K-12® strain.

